Tacrolimus lowers the body's ability to fight an infection/disease (immunosuppression). This may increase your risk of developing an infection or certain types of cancer (such as skin cancer, lymphoma). To reduce the risk of these serious side effects, take this medication at the lowest effective dose as directed by your doctor. Keep all medical and lab appointments.
Tell your doctor right away if you develop any of the following: unusual skin changes, change in the appearance/size of moles, unusual growths/lumps, swollen glands, night sweats, unexplained weight loss, signs of infection (such as sore throat that doesn't go away, fever).
Who should not take Tacrolimus?
Show MoreUses
Tacrolimus is used with other medications to prevent rejection of a kidney, heart, liver, or lung transplant. This medication belongs to a class of drugs known as immunosuppressants. It works by weakening your body's defense system (immune system) to help your body accept the new organ as if it were your own.
How to use Tacrolimus
Read the Patient Information Leaflet if available from your pharmacist before you start taking tacrolimus and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth with or without food as directed by your doctor, usually every 12 hours. If you have nausea or an upset stomach, you may take this drug with food, although this may cause your body to absorb less of the drug. However, you must choose one way (with food or without food) and always take this medication the same way so that your body always absorbs the same amount of drug. Consult your doctor or pharmacist for more details.
If you are taking the capsules, swallow them whole. Do not open or crush the capsules.
If you are using the granules, read the manufacturer's Instructions for Use first. Use only non-plastic cups, spoons, or oral syringes when preparing or taking a dose. Do not sprinkle the granules on food. Open up the prescribed number of packets for your dose and mix the granules in a cup with 1 to 2 tablespoons (15 to 30 milliliters) of room temperature water and stir well. Drink the dose immediately. You may use an oral syringe to give the dose. Rinse the cup or syringe with the same amount of water and drink the rinse water to make sure the complete dose is taken. Do not prepare the granule mixture ahead of time. Do not save any of the mixture for later use.
The dosage is based on your weight, medical condition, lab tests (such as tacrolimus trough levels), and response to treatment.
Tacrolimus is available in different formulations (such as immediate and extended-release). Do not switch between different forms of tacrolimus without consulting your doctor.
Do not increase your dose or take this medication more often without your doctor's approval. Your condition will not improve any faster and the risk of serious side effects may be increased. Also, do not stop taking this medication without your doctor's approval.
Take this medication regularly in order to get the most benefit from it. It is important to take all doses on time to keep the amount of medicine in your body at a constant level. Remember to take it at the same times each day.
Avoid eating grapefruit or drinking grapefruit juice while using this medication unless your doctor or pharmacist says you may do so safely. Grapefruit can increase the chance of side effects with this medicine. Ask your doctor or pharmacist for more details.
Since this drug can be absorbed through the skin and lungs and may harm an unborn baby, women who are pregnant or who may become pregnant should not handle this medication or breathe the dust from the capsules.
Tell your doctor if your condition worsens.
Side Effects
See also Warning section.
Shaking, headache, diarrhea, nausea/vomiting, upset stomach, loss of appetite, trouble sleeping, and numbness/tingling of the hands/feet may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: mental/mood changes, dizziness, signs of kidney problems (such as change in the amount of urine), pounding heartbeat, symptoms of heart failure (such as shortness of breath, swelling ankles/feet, unusual tiredness, unusual/sudden weight gain), hearing problems (such as hearing loss, ringing in the ears), pain/redness/swelling of arms or legs, easy bruising/bleeding, muscle pain/cramp/weakness, signs of liver problems (such as nausea/vomiting that doesn't stop, yellowing eyes/skin, dark urine, stomach/abdominal pain), severe leg pain.
This medication may also increase your risk of getting a rare but very serious (sometimes fatal) brain infection (progressive multifocal leukoencephalopathy-PML). Get medical help right away if any of these rare but very serious side effects occur: clumsiness, loss of coordination, weakness, sudden change in your thinking (such as confusion, difficulty concentrating), difficulty moving your muscles, problems with speech, seizure, vision changes.
Get medical help right away if you have any very serious side effects, including: fainting, fast/irregular heartbeat, severe dizziness, chest/jaw/left arm pain, black stools, vomit that looks like coffee grounds.
This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high. Your doctor may control your blood pressure with medication.
Tacrolimus may cause diabetes. Tell your doctor or pharmacist if you experience any of the following symptoms of high blood sugar: increased thirst/hunger, frequent urination.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US -
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before taking tacrolimus, tell your doctor or pharmacist if you are allergic to it; or to other macrolide medications (such as sirolimus); or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: mineral imbalances (such as high potassium), kidney disease, any recent/current infections, cancer, liver disease, high blood pressure, diabetes.
Tacrolimus may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.
The risk of QT prolongation may be increased if you have certain medical conditions or are taking other drugs that may cause QT prolongation. Before using tacrolimus, tell your doctor or pharmacist of all the drugs you take and if you have any of the following conditions: certain heart problems (heart failure, slow heartbeat, QT prolongation in the EKG), family history of certain heart problems (QT prolongation in the EKG, sudden cardiac death).
Low levels of potassium or magnesium in the blood may also increase your risk of QT prolongation. This risk may increase if you use certain drugs (such as diuretics/"water pills") or if you have conditions such as severe sweating, diarrhea, or vomiting. Talk to your doctor about using tacrolimus safely.
This medication may increase your risk of developing skin cancer. Limit your time in the sun. Avoid tanning booths and sunlamps. Use sunscreen and wear protective clothing when outdoors.
Tacrolimus can make you more likely to get infections or may make current infections worse. Stay away from anyone who has an infection that may easily spread (such as chickenpox, COVID-19, measles, flu). Talk to your doctor if you have been exposed to an infection or for more details.
Tell your health care professional that you are using tacrolimus before having any immunizations/vaccinations. Avoid contact with people who have recently received live vaccines (such as flu vaccine inhaled through the nose).
This drug may increase your potassium levels. Before using potassium supplements or salt substitutes containing potassium, consult your doctor or pharmacist.
Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).
Older adults may be more sensitive to the side effects of this drug, especially QT prolongation (see above).
Since this drug can be absorbed through the skin and lungs and may harm an unborn baby, women who are pregnant or who may become pregnant should not handle this medication or breathe the dust from the capsules.
Tell your doctor if you are pregnant or plan to become pregnant. You should not become pregnant while using tacrolimus. Tacrolimus may harm an unborn baby. Men and women using this medication should ask about reliable forms of birth control before and during treatment. If you or your partner becomes pregnant, talk to your doctor right away about the risks and benefits of this medication.
This drug passes into breast milk and the effect on a nursing infant is unknown. Consult your doctor before breastfeeding.
Interactions
See also How to Use section.
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Some products that may interact with this drug include: aluminum/magnesium antacid, cyclosporine, sirolimus, temsirolimus, ziprasidone, other drugs that may increase the level of potassium in the blood (such as "water pills" including amiloride, spironolactone), other drugs that weaken the immune system/increase the risk of infection (such as natalizumab, rituximab).
Other medications can affect the removal of tacrolimus from your body, which may affect how tacrolimus works. Examples include cimetidine, danazol, nefazodone, ethinyl estradiol, methylprednisolone, St. John's wort, azole antifungals (such as itraconazole, voriconazole), HIV protease inhibitors (such as nelfinavir), rifamycins (such as rifampin, rifabutin), ritonavir, certain anti-seizure drugs (such as phenobarbital, phenytoin), among others.
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
Notes
Lab and/or medical tests (such as potassium levels, blood pressure, blood sugar, tacrolimus trough level, kidney/liver function) will be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
If you have had an organ transplant, it is recommended that you attend a transplant education class or support group. Learn the signs of organ rejection such as a feeling of being ill, fever, or tenderness/pain around the transplanted organ. Tell your doctor right away if you notice any of these signs.
Missed Dose
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
Storage
Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.Information last revised March 2024. Copyright(c) 2024 First Databank, Inc.
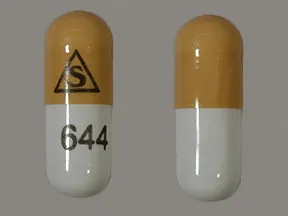


- color
- white,brown
- shape
- oblong
- imprint
- logo, 644
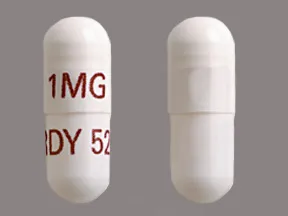


- color
- white
- shape
- oblong
- imprint
- 1MG, RDY 526
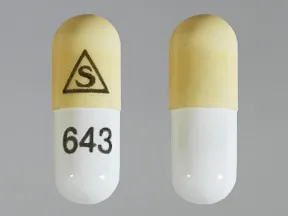


- color
- ivory,white
- shape
- oblong
- imprint
- logo, 643
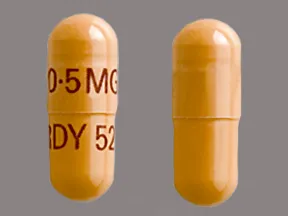


- color
- dark yellow
- shape
- oblong
- imprint
- 0.5MG, RDY 525
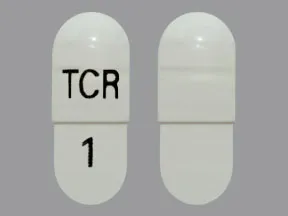


- color
- white
- shape
- oblong
- imprint
- TCR, 1
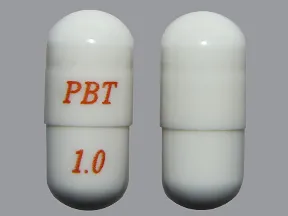


- color
- white
- shape
- oblong
- imprint
- PBT, 1.0



- color
- dark grayish red
- shape
- oblong
- imprint
- 5MG, RDY 527
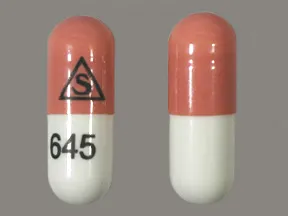


- color
- orange,white
- shape
- oblong
- imprint
- logo, 645



- color
- light orange,gray
- shape
- oblong
- imprint
- MYLAN 2045, MYLAN 2045



- color
- teal
- shape
- oblong
- imprint
- SAL, 722
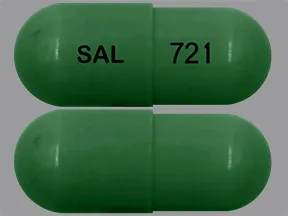


- color
- green
- shape
- oblong
- imprint
- SAL, 721



- color
- light blue,gray
- shape
- oblong
- imprint
- MYLAN 2046, MYLAN 2046
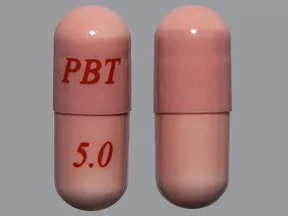


- color
- pink
- shape
- oblong
- imprint
- PBT 5.0



- color
- ruby red,gray
- shape
- oblong
- imprint
- MYLAN 2047, MYLAN 2047



- color
- light yellow
- shape
- oblong
- imprint
- PBT, 0.5



- color
- white
- shape
- oblong
- imprint
- logo, 686



- color
- light yellow
- shape
- oblong
- imprint
- logo, 685



- color
- white
- shape
- oblong
- imprint
- 1mg, Tacro



- color
- yellow
- shape
- oblong
- imprint
- 0.5mg, Tacro
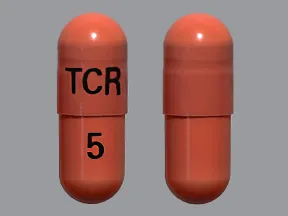


- color
- pink
- shape
- oblong
- imprint
- TCR, 5
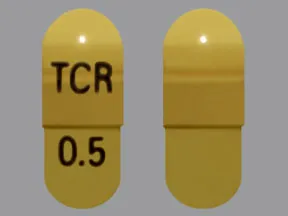


- color
- light yellow
- shape
- oblong
- imprint
- TCR, 0.5

- color
- white,brown
- shape
- oblong
- imprint
- logo, 644

- color
- white
- shape
- oblong
- imprint
- 1MG, RDY 526

- color
- ivory,white
- shape
- oblong
- imprint
- logo, 643

- color
- dark yellow
- shape
- oblong
- imprint
- 0.5MG, RDY 525

- color
- white
- shape
- oblong
- imprint
- TCR, 1

- color
- white
- shape
- oblong
- imprint
- PBT, 1.0

- color
- dark grayish red
- shape
- oblong
- imprint
- 5MG, RDY 527

- color
- orange,white
- shape
- oblong
- imprint
- logo, 645

- color
- light orange,gray
- shape
- oblong
- imprint
- MYLAN 2045, MYLAN 2045

- color
- teal
- shape
- oblong
- imprint
- SAL, 722

- color
- green
- shape
- oblong
- imprint
- SAL, 721

- color
- light blue,gray
- shape
- oblong
- imprint
- MYLAN 2046, MYLAN 2046

- color
- pink
- shape
- oblong
- imprint
- PBT 5.0

- color
- ruby red,gray
- shape
- oblong
- imprint
- MYLAN 2047, MYLAN 2047

- color
- light yellow
- shape
- oblong
- imprint
- PBT, 0.5

- color
- white
- shape
- oblong
- imprint
- logo, 686

- color
- light yellow
- shape
- oblong
- imprint
- logo, 685

- color
- white
- shape
- oblong
- imprint
- 1mg, Tacro

- color
- yellow
- shape
- oblong
- imprint
- 0.5mg, Tacro

- color
- pink
- shape
- oblong
- imprint
- TCR, 5

- color
- light yellow
- shape
- oblong
- imprint
- TCR, 0.5
Drug Survey
Are you taking Tacrolimus?
Are you considering switching to Tacrolimus?
How satisfied are you with the results?
Are you planning to see a doctor about switching your medication?
How long have you been taking Tacrolimus?
Are you planning to see a doctor about switching your medication?
Thanks for taking our survey!
Recommended For You
Find a doctor near you